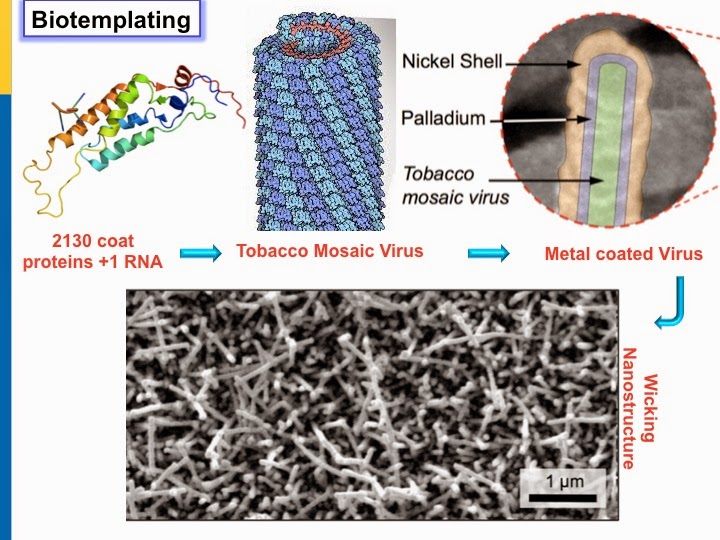
Art or Science?
Are these pastel fractals the creation of an avant garde artist from some postmodern cubism movement? You may be surprised to learn that these are high resolution images of bacterial populations growing on a petri dish!
◈ Bacterial Art: First, the familiar E. coli bacteria were genetically marked with differently colored fluorescent proteins before mixing together on an agar plate. Each rod-shaped bacterium grows by division to give a single file of cells that is sensitive to small mechanical forces from neighboring cells pushing and jostling against each other. The line of cells buckles in a way that is predicted by fractal mathematics. As the bacteria grow to form a confluent film, jagged boundaries emerge between differently colored clonal lines. Zooming in, the patterns are self-similar, repeating at scales from millimeters to micrometers! Mutant bacteria that form spherical cells don’t produce these fractal patterns.
◈ Form and Function: What do these beautiful images teach us? They help us understand how patterning happens on a nanoscale. In synthetic biology the goal is to engineer populations of cells to produce spatial patterns, synchronized signals and predictable behavior that can be simulated using simple, mathematically coded rules.
◈ Life Imitates Art? Oscar Wilde reversed the conventional when he claimed that life imitates art far more than art imitates life. What do you think he meant by this? It seems to me that this bacterial fractal “art” perfectly illustrates John Berger’s definition of Cubism: “The metaphorical model of Cubism is the diagram: The diagram being a visible symbolic representation of invisible processes, forces, structures.”
Reference (and more beautiful images): http://data.plantsci.cam.ac.uk/Haseloff/resources/LabPapers/Rudge2013.pdf
#ScienceSunday