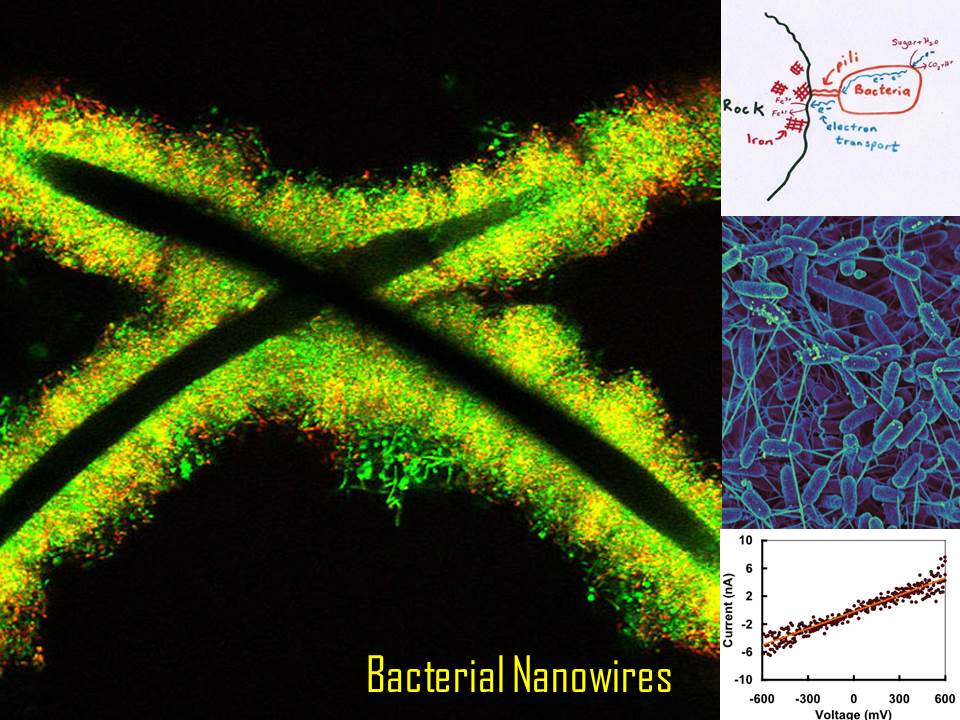
Bio-Nanowires Conduct Electricity
▶ Imagine a conducting nanowire, only 3-5 nm wide but many thousand times longer, connecting a microbial community to form mini-power grids. Naturally occurring soil bacteria, such as Geobacter, use these conductive pili for long-range electron transport. How and why do they do this?
▶ All living organisms respire. Our cells break down sugars to obtain energy by extracting electrons that are handed down a relay chain to oxygen, which becomes water. The proteins (cytochromes) that conduct electrons are aided by special metallic centers, studded with iron, so they can cycle between Fe2+ (ferrous) and Fe3+ (ferric) states that differ by one electron. Geobacter uses these cytochromes too, just as our cells do. But oxygen only made its debut a mere 2.4 billion years ago. Before that, ancient bacteria shuttled the electrons to other acceptors, such as sulfides, nitrates and Fe3+. When Geobacter is deprived of oxygen, it grows out long pili into mineral rich rocks and “breathes” iron (drawing on top right). The current is believed to pass between layers of bacteria (middle right image) across a distance of 12 millimetres, which may not seem large, but is 10,000 body lengths to bacteria!
▶ But can proteins conduct current? Researchers knew that the pili were conductive, behaving like ohmic devices (image at bottom right). Although the pili were decorated with cytochromes, they were spaced too far apart to transfer electrons between their metallic centers. When the protein chains were mutated to replace a type of amino acid, the pili lost conductivity. These “aromatic” amino acids have pi-pi orbitals that may be conducting electrons.
▶ Live Wires: Bacterial nanowires can be used in generating microbial energy cells, bioremediation of pollutants (like uranium), and in nano-manufacturing of a variety of devices. The main image shows bacteria growing on metal electrodes.
⚛ Ref: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3604773/
⚛ Sources: http://www.geobacter.org/Nanowires, http://goo.gl/E6Kpf , http://goo.gl/si6aH , http://goo.gl/T76L5
#ScienceEveryday when it’s not #ScienceSunday .

Biological wires (busses) is a subject I am very interested in in connection with my engineering of a biological microchip. Thus, thanks so much for this share Rajini Rao
How does static electricity or gravity affect nanowires?
They are not as conductive as copper wires, of course, but in the same order as semiconductors, I believe. I hope to see much more in the way of applications in the future, Gerd Moe-Behrens .
Julie Ware Mark Art Productions , I don’t think gravity would have any effect on these but static electricity could disrupt the conductance momentarily. Although these function in a grounded state..that is, they are so conductive, that I expect that static electricity would not build up significantly. Did you have a particular reason for asking?
Rajini Rao
I am not sceintifically oriented but curious about a lot of things. It does seem that static electricity, even a high degree in clothing can change a persons energy levels . . . leading me to believe that it must in some way affect our cells. Is there any long term effects?
I hope so Rajini Rao In biological computers are wires often chemical wires. You might like: Robust multicellular computing using genetically encoded NOR gates and chemical ‘wires’ http://www.nature.com/nature/journal/v469/n7329/full/nature09565.html
Gerd Moe-Behrens , thanks! I’ve not seen that paper..I did see the one by Drew Endy in Science. I thought the video explained it very well and I wanted to share it in the Science on Google+ community if you have not already.
Static electricity generated on people wearing nylons for example is really tiny, Julie Ware Mark Art Productions . I’m not aware of any significant findings that have been reported, but I’ll let you know if I find anything.
In semiconductors, such as those used in transistor technology, a gate ( like in FET) is usually used to control electron flow. Wondering what control that flow across the lipi ?
Talking of molecular machinery, did you and Gerd Moe-Behrens see Vadilson Malaquias dos Santos’s tiny turnstile post?: http://goo.gl/MKBxG
Tom Lee , in these bacteria, current flow is simply determined by electron donor and acceptor. However, there are advances in synthetic biology, to design logic gates that function in bacterial cells. These switches could be combined with electrical bio-wires to make a transistor.
Thanks, Joe Dunlavy . I try to put in enough detail to make the science meaningful but still accessible. Sometimes, I succeed more than other times 🙂
Recent studies have greatly expanded the range of microorganisms known to function either as electrode-reducing microorganisms at the anode or as electrode-oxidizing microorganisms at the cathode. Microorganisms that can completely oxidize organic compounds with an electrode serving as the sole electron acceptor are expected to be the primary contributors to power production ( Aromatic amino acids are the most likely source of pi orbital stacking in a protein filament such as pili). The mechanisms for long-range extracellular electron transport in Geobacter species are of interest because of the important role that Geobacter species play in the biogeochemistry of a diversity of anaerobic soils and sediments and in several bioenergy strategies. Extracellular electron transfer by Geobacter species plays an important role in the biogeochemistry of soils and sediments and has a number of bioenergy applications and new technologies . For example, microbial reduction of Fe(III) oxide is one of the most geochemically significant processes in anaerobic soils, aquatic sediments, and aquifers, and Geobacter organisms are often abundant in such environments.
Very good… 😉
Rajini Rao I did. see https://plus.google.com/115722829851477319854/posts/M4u3MaitqWH?hl=en see comments
Thanks, Vadilson Malaquias dos Santos . If anyone wants to read the entire article by Derek Lovley, here it is: http://www.microbialfuelcell.org/Publications/EBC/Current%20Opinion-Microbe%20Electric.pdf
Thanks, Gerd Moe-Behrens . I had a question about their method that I left on your post.
I answered it there.
Why would biological conductors attract application?
Because they can function in places that synthetic conductors cannot. They can be cheap, easy to grow and renewable. These bacteria are also naturally adept at geochemical conversion of compounds and at bioremediation in large areas of contaminated soil or water. The articles in the links list the advantages more comprehensively.
So how do the bacteria ‘breathe’ iron? There must be some reaction “Fe + stuff -> metabolites + energy” What’s stuff? What are the metabolites?
Totally fascinating, awesome post Rajini Rao 😉
fascinating
Linas Vepstas , the cartoon on the upper right of the image is a simplified summary. The initial biochemical steps of breaking down carbon compounds, like sugars, are the same. In the course of this process, electrons are removed (in our mitochondria, these electrons go onto mobile carriers like NADH and then to the electron transport chain). The difference lies in the terminal (final) acceptor for electrons. In aerobic respiration, it is molecular oxygen, which becomes reduced and combines with H+ to form water. In this case, it is Fe3+ which becomes reduced to Fe2+. Other compounds may also be acceptors.
OK, I had to study wikipedia .. so if I understand this properly, the rock is hematite (ferric oxide)? But reducing that iron now leaves a reduced oxygen floating around, which eventually finds a proton, or some carbon to form carbon dioxide? … while the rock is converted to magnetite, right? OK, I think I’m starting to get it. The other Fe3+ mineral that I can find is ferric sulfate, but reducing that would leave behind sulfuric acid, which doesn’t seem healthy.
awesome topic. How does the organism determine if it attracted to anode or cathode — I am thinking maybe by absorbing elements that are + or – ions? Can this be used as an organic battery (might be very low voltage)?
I’m thinking that this could be the precursor to the full fledged realization of the themes and topics discussed in the Matrix :-p
Apologies for the delay in getting back to the comments (busy with yesterday’s Hang out on the genome and ENCODE project). Linas Vepstas , you’re right..and the geochemical conversions can be quite complex depending on what reactants are available. Keep in mind that most of these occur in anaerobic conditions, when oxygen is not available.
Jim Gorycki the organism serves as the negative electrode because it generates electrons. Yes, it can serve as a microbial cell or bug powered battery..I saw an image of a toy truck powered by these but I can’t find it now. Here is a short clip: Bug Powered Batteries
Rajini Rao Vadilson Malaquias dos Santos thanks for the info and the links. Awesome!
nice